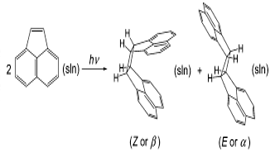
Energetics of the Thermal Dimerization of Acenaphthylene to Heptacyclene
J. Phys. Chem. A 2006, 110, 2299-2307. DOI: 10.1021/jp056275o
Abstract
The energetics of the thermal dimerization of acenaphthylene to give Z- or E-heptacyclene was investigated. The standard molar enthalpy of the formation of monoclinic Z- and E-heptacyclene isomers at 298.15 K was determined as ∆fHom (E-C24H16, cr) = 269.3 ± 5.6 kJ·mol-1 and ∆fHom (Z-C24H16, cr) = 317.7 ± 5.6 kJ·mol-1, respectively, by microcombustion calorimetry. The corresponding enthalpies of sublimation, ∆subHom (E-C24H16) = (149.0 ± 3.1) kJ·mol-1 and ∆subHom (Z-C24H16) = (128.5 ± 2.3) kJ·mol-1 were also obtained by Knudsen effusion and Calvet-drop microcalorimetry methods, leading to ∆fHom (E-C24H16, g) = (418.3 ± 6.4) kJ·mol-1 and ∆fHom (Z-C24H16, g) = (446.2 ± 6.1) kJ·mol-1, respectively. These results, in conjunction with the reported enthalpies of formation of solid and gaseous acenaphthylene, and the entropies of acenaphthylene and both hepatcyclene isomers obtained by the B3LYP/6-31G(d,p) method led to the conclusion that at 298.15 K the thermal dimerization of acenaphthylene is considerably exothermic and exergonic in the solid and gaseous states (although more favorable when the E isomer is the product), suggesting that the nonobservation of the reaction under these conditions is of kinetic nature. A full determination of the molecular and crystal structure of the E dimer by X-ray diffraction is reported for the first time. Finally, molecular dynamics computer simulations on acenaphthylene and the heptacyclene solids were carried out and the results discussed in light of the corresponding structural and ∆subHom data experimentally obtained.
Return Next