Comparative Study of Al-MCM Materials Prepared at Room Temperature with Different Aluminium Sources and by some Hydrothermal Methods
Micropor. Mesopor. Mater. 2006, 92, 270–285. DOI: 10.1016/j.micromeso.2006.01.010
Abstract
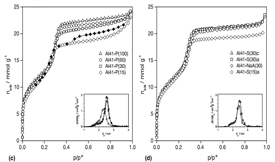
A comparison of the pore structural properties and catalytic activity of MCM-41 containing aluminium, prepared at room temperature with different aluminium sources, is presented. In addition, they are compared with those of MCM-41 obtained by two conventional hydrothermal procedures and room temperature synthesised Al-MCM-48 grades. Al-MCM-41 samples were prepared by direct synthesis at room temperature with tetraethoxysilane (TEOS) and aluminium isopropoxide, aluminium sulfate or sodium aluminate, and by two hydrothermal routes using silica Ludox suspension and sodium aluminate (at 377 K) or Cab-O-Sil silica and alumina (at 377 K and 423 K). The synthesis of MCM-48 grades was also carried out at room temperature with TEOS and aluminium sulfate or nitrate. The materials were characterised by X-ray diffraction, nitrogen adsorption at 77 K and solid-state 27Al MAS NMR. The catalytic activity was evaluated in the reaction of double bond position isomerisation of 1-butene to 2-butenes at 373 K. It is concluded that the room temperature synthesis method used resulted in the preparation in a short period of time of well structured MCM-41 materials that contained predominantly tetracoordinated aluminium and presented acidic catalytic activity similar to or better than those prepared by the two hydrothermal procedures. Aluminium sulfate is a good alternative to isopropoxide in the room temperature synthesis as it resulted in samples with very uniform pore size, good hexagonal ordering and increasing catalytic performance at least up to Si/Al of 13. The most active sample was a MCM-48 grade also prepared at room temperature with the same precursor.