Energetics of C-F, C-Cl, C-Br, and C-I Bonds in 2-Haloethanols. Enthalpies of Formation of XCH2CH2OH (X ) F, Cl, Br, I) Compounds and of the 2-Hydroxyethyl Radical
C.E.S. Bernardes, M.E. Minas da Piedade, L.M.P.F. Amaral, A.I.M.C.L. Ferreira, M.A.V. Ribeiro da Silva, H.P. Diogo, B.J.C. Cabral
J. Phys. Chem. A 2007, 111, 1713-1720. DOI: 10.1021/jp0675678
Abstract
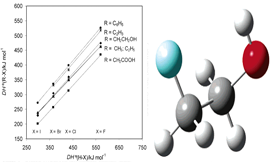
The energetics of the C−F, C−Cl, C−Br, and C−I bonds in 2-haloethanols was investigated by using a combination of experimental and theoretical methods. The standard molar enthalpies of formation of 2-chloro-, 2-bromo-, and 2-iodoethanol, at 298.15 K, were determined as ΔfHom (ClCH2CH2OH, l) = −315.5 ± 0.7 kJ/mol, ΔfHom (BrCH2CH2OH, l) = −275.8 ± 0.6 kJ/mol, ΔfHom (ICH2CH2OH, l) = −207.3 ± 0.7 kJ/mol, by rotating-bomb combustion calorimetry. The corresponding standard molar enthalpies of vaporization, ΔvapHom (ClCH2CH2OH) = 48.32 ± 0.37 kJ/mol, ΔvapHom (BrCH2CH2OH) = 54.08 ± 0.40 kJ/mol, and ΔvapHom (ICH2CH2OH) = 57.03 ± 0.20 kJ/mol were also obtained by Calvet-drop microcalorimetry. The condensed phase and vaporization enthalpy data lead to ΔfHom (ClCH2CH2OH, g) = −267.2 ± 0.8 kJ/mol, ΔfHom (BrCH2CH2OH, g) = −221.7 ± 0.7 kJ/mol, and ΔfHom (ICH2CH2OH, g) = −150.3 ± 0.7 kJ/mol. These values, together with the enthalpy of selected isodesmic and isogyric gas-phase reactions predicted by density functional theory (B3LYP/cc-pVTZ) and CBS-QB3 calculations were used to derive the enthalpies of formation of gaseous 2-fluoroethanol, ΔfHom (FCH2CH2OH, g) = −423.6 ± 5.0 kJ/mol, and of the 2-hydroxyethyl radical, ΔfHom (CH2CH2OH, g) = −28.7 ± 8.0 kJ/mol. The obtained thermochemical data led to the following carbon−halogen bond dissociation enthalpies: DHo(X−CH2CH2OH) = 474.4 ± 9.4 kJ/mol (X = F), 359.9 ± 8.0 kJ/mol (X = Cl), 305.0 ± 8.0 kJ/mol (X = Br), 228.7 ± 8.1 kJ/mol (X = I). These values were compared with the corresponding C−X bond dissociation enthalpies in XCH2COOH, XCH3, XC2H5, XCHCH2, and XC6H5. In view of this comparison the computational methods mentioned above were also used to obtain ΔfHom (FCH2COOH, g) = −594.0 ± 5.0 kJ/mol from which DHo(F−CH2COOH) = 435.4 ± 5.4 kJ/mol. The order DHo(C−F) > DHo(C−Cl) > DHo(C−Br) > DHo(C−I) is observed for the haloalcohols and all other RX compounds. It is finally concluded that the major qualitative trends exhibited by the C−X bond dissociation enthalpies for the series of compounds studied in this work can be predicted by Pauling's electrostatic-covalent model.
Return Previous Next