Polymorphic Phase Transition in 4'-Hydroxyacetophenone: Equilibrium Temperature, Kinetic Barrier and the Relative Stability of Z' = 1 and Z' = 2 Forms
A. Joseph, C.E.S. Bernardes, A.I. Druzhinina, R.M. Varushchenko, T.Y. Nguyen, F. Emmerling, L. Yuan, V. Dupray, G. Coquerel, M.E. Minas da Piedade
Cryst. Growth Des. 2017, 17, 1918−1932.
Abstract
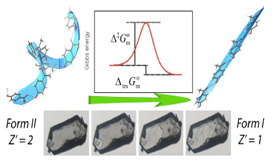
Particularly relevant in the context of polymorphism is understanding how structural, thermodynamic, and kinetic factors dictate the stability domains of polymorphs, their tendency to interconvert through phase transitions, or their possibility to exist in metastable states. These three aspects were investigated here for two 4'-hydroxyacetophenone (HAP) polymorphs, differing in crystal system, space group and, number and conformation of molecules in the asymmetric unit. The results led to ΔfGom - T phase diagram highlighting the enantiotropic nature of the system and the fact that the Z' = 1 polymorph is not necessarily more stable than its Z' = 2 counterpart. It was also shown that the form II → form I transition is entropy driven and is likely to occur through a nucleation and growth mechanism, which does not involve intermediate phases, and is characterized by a high activation energy. Finally, although it has been noted that conflicts between hydrogen bond formation and close packing are usually behind exceptions from the hypothesis of Z' = 1 forms being more stable than their higher Z' analogues, in this case, the HAP polymorph with stronger hydrogen bonds (Z' = 2) is also the one with higher density.
Return Previous Next