Linking Aggregation in Solution, Solvation, and Solubility of Simvastatin: An Experimental and MD Simulation Study
R.G. Simões, P.L.T. Melo, C.E.S. Bernardes, M.T. Heilmann, F. Emmerling, M.E. Minas da Piedade
Cryst. Growth Des. 2021, 21, 544-551.
Abstract
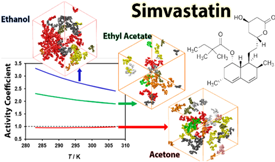
The solubility is generally thought to be higher if the solvent effectively solvates solute molecules that are well-separated from each other. The present work suggests, however, that the formation of large solute aggregates does not necessarily imply less effective solvation and lower solubility. Measurements of the solubility of simvastatin (one of the most commonly prescribed antihyperlipidemic drugs) in three solvents with different polarities and protic characters, led to the solubility order acetone > ethyl acetate > ethanol, in the full temperature range covered by the experiments (283−308 K). An analysis of the structures of the different solutions on the basis of molecular dynamics simulation results indicated that this trend seems to be determined by a balance between the solute tendency toward aggregation and the ability of the solvent to efficiently solvate it, by integrating the cluster structures, regardless of their size, and the effective establishment of solvent−solute interactions.
Return Previous Next