DMSO/IL Solvent Systems for Cellulose Dissolution: Binary or Ternary Mixtures?
T.G. Paiva, M. Zanatta, E.J. Cabrita, C.E.S. Bernardes, M.C. Corvo
J. Mol. Liq. 2022, 345, 117810.
Abstract
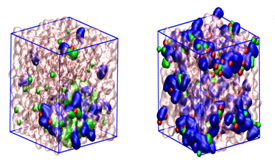
The mechanism of cellulose dissolution in ionic liquid (IL)/dimethyl sulfoxide (DMSO) solvent systems has attracted much attention due to the possible replacement of synthetic materials. However, the solvent behaviour is not completely understood. This work has found an explanation for the solvent behaviour in cellulose dissolution, considering the almost unavoidable presence of the water. Ternary [C4mim] Cl/DMSO/H2O mixtures were studied with Nuclear Magnetic Resonance experiments and molecular dynamics simulations to explore IL/molecular solvents interactions and disclose the water interactions in these complex media. Titration of binary and ternary solvent systems with water and DMSO disclosed a relation between water’s proton chemical shift and the molar fraction of the mixture components, creating an unprecedent theory to predict the cellulose solvation ability. A ‘‘working range” for IL/DMSO/H2O ratio was observed, tested in cellulose dissolution, and rationalized using cellobiose interaction. Within this solvent ratio, the interactions between components are maximized, being switched on, while out of the range, the interactions are no longer detected.
Return Previous Next