A Robust yet Metastable New Hemihydrate of 4-Hydroxynicotinic Acid
Cryst. Growth Des. 2011, 11, 2803–2810. DOI: 10.1021/cg1015687
Abstract
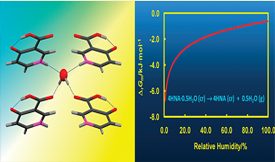
A new hydrate of 4-hydroxynicotinic acid (4HNA), orthorhombic, space group P21212, was isolated and characterized. The stoichiometry 4HNA·0.5H2O was established both from the determination of the crystal structure by single-crystal X-ray diffraction at 296 K and from the results of thermogravimetic experiments. The packing analysis revealed that lattice water molecules are isolated from each other (isolated site hydrate) and form hydrogen bonds with four independent 4HNA units: two of the NH···O type, as the acceptor and two others of OH···OC type, as donor. A thermodynamic analysis of the driving force for water loss from 4HNA·0.5H2O, based on calorimetric results, suggested that the reaction is exergonic (ΔrGm < 0) at 298.15 K, even for a relative humidity of 100%. Hence, the experimentally observed robustness of the hydrate toward spontaneous dehydration under ambient temperature, pressure, and humidity conditions must be due to the existence of a large activation barrier for the removal of the water molecules from the crystal lattice.
Return Previous Next