The Structure of Aqueous Solutions of a Hydrophilic Ionic Liquid: The Full Concentration Range of 1-Ethyl-3-methylimidazolium Ethylsulfate and Water
J. Phys. Chem. B 2011, 115, 2067–2074. DOI: 10.1021/jp1113202
Abstract
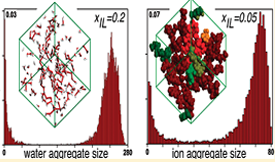
Several structural features of aqueous solutions of the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate were analyzed in the entire concentration range using molecular dynamics simulation results. Different analysis tools developed in-house were applied to describe the size and connectivity of different water and ion aggregates as a function of the solution concentration. Four concentration ranges—xH2O < 0.5, 0.5 < xH2O < 0.8, 0.8 < xH2O < 0.95, and xH2O > 0.95—with four distinct structural regimes—isolated water molecules, chain-like water aggregates, bicontinuous system, and isolated ions or small ion clusters — were identified and discussed, including two different percolation limits: that of water in the ionic liquid network (around xH2O = 0.8) and that of the ionic liquid in water (around xH2O = 0.95).
Return Previous Next