High Ionicity Ionic Liquids (HIILs): Comparing the Effect of Ethylsulfonate and Ethylsulfate Anions
F.S. Oliveira, A.B.Pereiro, J.M.M. Araújo, C.E.S. Bernardes, J.N. Canongia Lopes, S. Todorovic, G. Feio, P.L. Almeida, L.P.N. Rebelo, I.M. Marrucho
Phys. Chem. Chem. Phys. 2013, 15, 18138-18147. DOI: 10.1039/c3cp52642a
Abstract
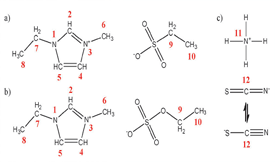
The subject of ionicity has been extensively discussed in the last decade, due to the importance of understanding the thermodynamic and thermophysical behaviour of ionic liquids. In our previous work, we established that ionic liquids' ionicity could be improved by the dissolution of simple inorganic salts in their milieu. In this work, a comparison between the thermophysical properties of two binary systems of ionic liquid + inorganic salt is presented. The effect of the ammonium thiocyanate salt on the ionicity of two similar ionic liquids, 1-ethyl-3-methylimidazolium ethylsulfonate and ethylsulfate, is investigated in terms of the related thermophysical properties, such as density, viscosity and ionic conductivity in the temperature range 298.15–323.15 K. In addition, spectroscopic (NMR and Raman) and molecular dynamic studies were conducted in order to better understand the interactions that occur at a molecular level. The obtained results reveal that although the two anions of the ionic liquid exhibit similar chemical structures, the presence of one additional oxygen in the ethylsulfate anion has a major impact on the thermophysical properties of the studied systems.
Return Previous Next