Nano-segregation in Ionic Liquids: Scorpions and Vanishing Chains
K. Shimizu, C.E.S. Bernardes, A. Triolo, J.N. Canongia Lopes
Phys. Chem. Chem. Phys. 2013, 15, 16256-16262. DOI: 10.1039/c3cp52357h
Abstract
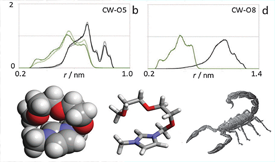
The present study analyses the large structural differences, first observed using X-ray diffraction, between 1-alkyl-3-methylimidazolium-based ionic liquids, [Cnmim][Ntf2] (n = 3, 6, 9), and their counterparts with ether-substituted alkyl side chains, [(C1OC1)(n/3)mim][Ntf2] (n = 3, 6, 9). The MD simulations—obtained using a non-polarizable atomistic force-field to model the ionic liquids under discussion—demonstrate that the suppression of the nanostructured nature in the ionic liquids with ether chains is persistent along the entire series and it is not due to any modification of the polar network of the ionic liquid but rather due to the different morphologies of the non-polar regions that surround it. The modification of the non-polar regions—shift from bulky segregated domains in [Cnmim][Ntf2] to thin enveloping ones in [(C1OC1)(n/3)mim][Ntf2]—are caused by the inability of the oxygen-substituted alkyl side chains to pack effectively side by side, the existence of kinks along the chain that lead eventually to intra-molecular, scorpion-like interactions between the chains and the imidazolium ring, and by their stronger interactions with the cations of the polar network via the lone electron pairs of the ether oxygen atoms.
Return Previous Next