Thermochemistry of 1-alkylimidazoles
J. Vitorino, F. Agapito, C.E.S. Bernardes, M.E.M. Piedade
Abstract
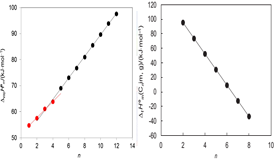
The standard molar enthalpies of vaporization, ∆vapHom, of 1-ethyl, 1-butyl, and 1-octylimidazole, at T = 298.15 K, were measured by Calvet-drop microcalorimetry. The values determined, together with results of high-level ab initio calculations (W1-F12 and CCSD(T)-F12) and published DvapHom data were used to obtain the enthalpies of formation in the gaseous and liquid states of 1-alkylimidazole compounds ranging from 1-methylimidazole to 1-dodecylimidazole. Structure-energetics trends related to the alkyl chain length are discussed.
Return Previous Next