Solvent Effects on the Polar Network of Ionic Liquid Solutions
Abstract
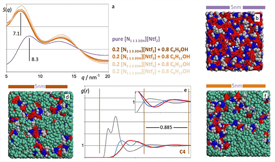
Molecular dynamics simulations were used to probe mixtures of ionic liquids (ILs) with common molecular solvents. Four types of systems were considered: (i) 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide plus benzene, hexafluorobenzene or 1,2-difluorobenzene mixtures; (ii) choline-based ILs plus ether mixtures (iii) choline-based ILs plus n-alkanol mixtures; and (iv) 1-butyl-3-methylimidazolium nitrate and 1-ethyl-3-methylimidazolium ethyl sulfate aqueous mixtures. The results produced a wealth of structural and aggregation information that highlight the resilience of the polar network of the ILs (formed by clusters of alternating ions and counter-ions) to the addition of different types of molecular solvent. The analysis of the MD data also shows that the intricate balance between different types of interaction (electrostatic, van der Waals, H-bond-like) between the different species present in the mixtures has a profound effect on the morphology of the mixtures at a mesoscopic scale. In the case of the IL aqueous solutions, the present results suggest an alternative interpretation for very recently published x-ray and neutron diffraction data on similar systems.
Return Previous Next