Solvation of Alcohols in Ionic Liquids – understanding the effect of the Anion and Cation
I.C.M. Vaz, M. Bastos, C.E.S. Bernardes, J.N. Canongia Lopes, L.M.N.B.F. Santos
Phys. Chem. Chem. Phys. 2018.
Abstract
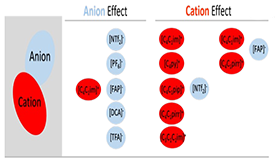
In this work we studied the effect of the anion and cation properties on the interaction of alcohols with ionic liquids (ILs), using propan-1-ol as a molecular probe. The enthalpies of solution at infinite dilution of propan-1-ol in several ILs were measured by Isothermal Titration Calorimetry (ITC). The calorimetric results were analysed together with molecular dynamics simulation and quantum chemical calculations of the interaction of the hydroxyl group of propan-1-ol with the anions. The results evidenced the role of the anion’s basicity on the intermolecular interactions of alcohols and ionic liquids and further revealed a secondary effect of the cation nature on the solvation process. The effect of the anion basicity on the strength of the interaction of alcohols with ionic liquids was evaluated by comparing results obtained for ILs with the same cation and different anions, [C4C1im][anion] (anions NTf2, PF6, FAP, DCA and TFA). The effect of the cation (size, aromaticity, charge distribution, acidity) was explored using five different cations of the NTf2 series, [Cation][NTf2] (cations C4C1im, C4C1pirr, C4py, and C4C1pip, C3C1C1im).
Return Previous Next