Comparative Structural Analyses in Four Ionic Liquid Systems: the Two Low-q Peaks of IL Structure Factor Functions
C.E.S. Bernardes, K. Shimizu, J.N. Canongia Lopes
Mol. Simul. 2018, 44, 478–484.
Abstract
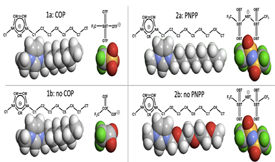
The unusual mesoscopic nature of ionic liquids can be investigated utilising the low-q range (2 ≤ q/nm-1 ≤ 18) of total and partial structure factor functions, S(q). The peak positions of the two most common low-q peaks – charged-ordering peak, COP and polar–non-polar peak, PNPP – are easy to interpret in terms of structural correlations within the IL systems. However, their relative intensities (existence or suppression) are not. In this study, molecular dynamics simulations were carried out to explore this issue for two pairs of ionic liquid systems: 1-octyl-3-methylimidazolium cations combined with either trifluorometylsulfonate or trifluorocarbonate anions; bis(trifluoromethylsulfonyl)imide anions combined either with 1-nonyl-3-methylimidazolium or 1-diglyme-3-methylimidazolium cations. The former pair compares similar systems with conspicuous or absent COPs; the latter pair contrasts two systems with prominent or suppressed PNPPs. In the frst case COP suppression is achieved by the replacement of a heavier atom (sulphur) by a lighter one (carbon) at the charged moiety of the anion, whereas in the second case, PNPP suppression is caused by a lower degree of segregation between polar and non-polar domains.
Return Previous Next