
This R-tool implements the Markov chain Monte Carlo and ordinary differential equation (ODE) hepatitis B virus (HBV) transmission model described in this research article. The core MCMC and ODE code is implemented in C/C++, and is wrapped with an R front end. This is not an R-package (although there are plans to extend the code and eventually make it into an R-package).
Abstract from publication in BMC Medicine
Sustainable Development Goals set a challenge for the elimination of hepatitis B virus (HBV) infection
as a public health concern by the year 2030. Deployment of a robust prophylactic vaccine and enhanced interventions
for prevention of mother to child transmission (PMTCT) are cornerstones of elimination strategy. However, in light of
the estimated global burden of 290 million cases, enhanced efforts are required to underpin optimisation of public
health strategy. Robust analysis of population epidemiology is particularly crucial for populations in Africa made
vulnerable by HIV co-infection, poverty, stigma and poor access to prevention, diagnosis and treatment.
We here set out to evaluate the current and future role of HBV vaccination and PMTCT as tools for
elimination. We first investigated the current impact of paediatric vaccination in a cohort of children with and
without HIV infection in Kimberley, South Africa. Second, we used these data to inform a new parsimonious model
to simulate the ongoing impact of preventive interventions. By applying these two approaches in parallel, we are able
to determine both the current impact of interventions, and the future projected outcome of ongoing preventive
strategies over time.
Existing efforts have been successful in reducing paediatric prevalence of HBV infection in this setting to
< 1%, demonstrating the success of the existing vaccine campaign. Our model predicts that, if consistently deployed,
combination efforts of vaccination and PMTCT can significantly reduce population prevalence (HBsAg) by 2030, such
that a major public health impact is possible even without achieving elimination. However, the prevalence of HBV
e-antigen (HBeAg)-positive carriers will decline more slowly, representing a persistent population reservoir. We show
that HIV co-infection significantly reduces titres of vaccine-mediated antibody, but has a relatively minor role in
influencing the projected time to elimination. Our model can also be applied to other settings in order to predict
impact and time to elimination based on specific interventions.
Through extensive deployment of preventive strategies for HBV, significant positive public health impact
is possible, although time to HBV elimination as a public health concern is likely to be
substantially longer than that proposed by current goals.
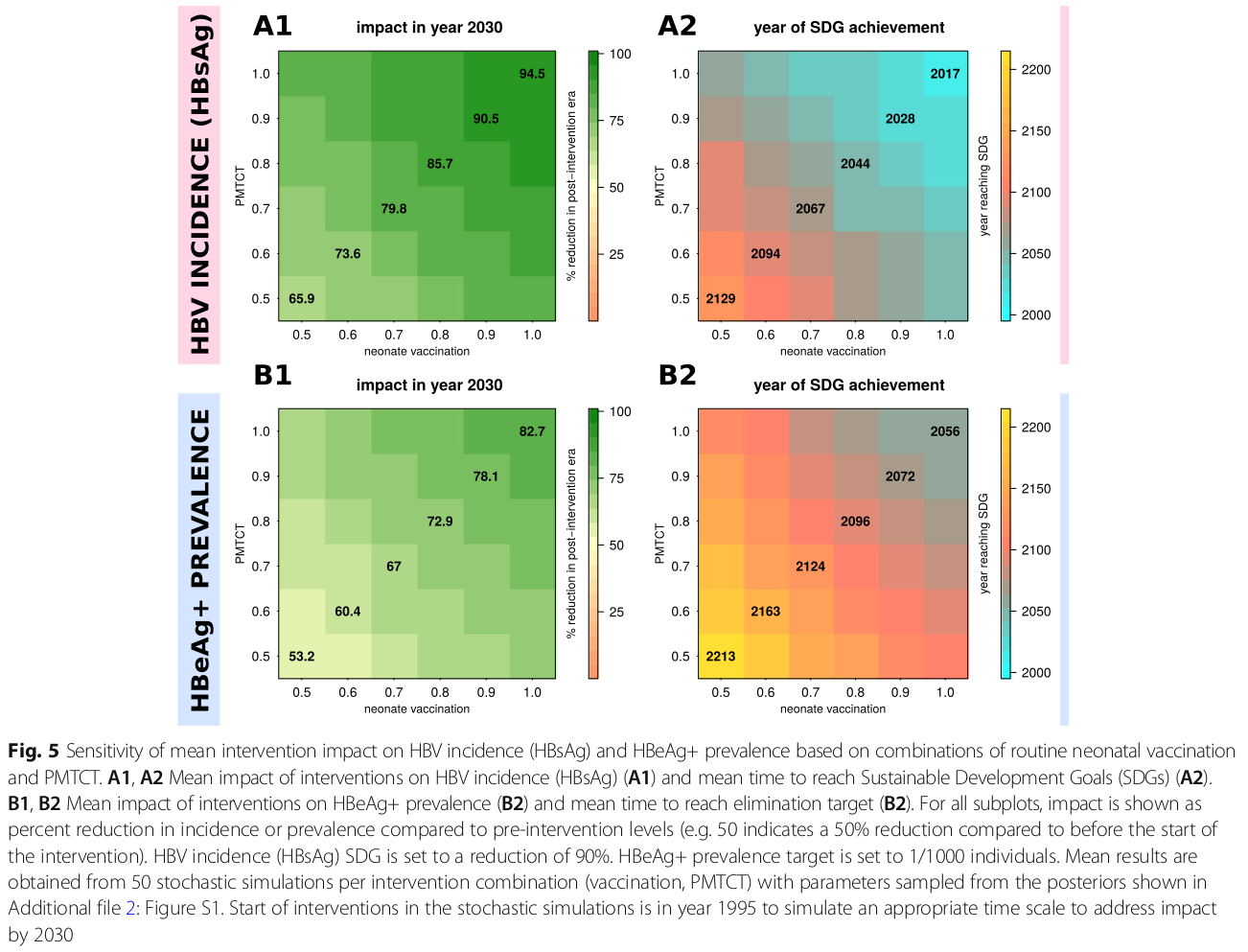
Summary of this figure: The left side of this figure presents the estimated reduction in HBV incidence (top) and HBeAg+ prevalence (bottom) by the year 2030. The right side presents the year in which the sustainable development goals (SDGs) would be reached. Each cell in the heatmaps presents the mean result of many stochastic simulations parameterised with different combinations of PMTCT and neonate vaccination (rows and columns of the heatmaps). More details in the academic publication.
Availability and requirements
The HBV control R-tool is available under a GNU GPL 3.0 license at SourceForge.

There you can find both a ZIP file with the code and a PDF with instructions can be found.
Newer versions and related materials will be deposited in such repository
and the reader should refer to it for further information and future changes.
This code is platform-independent requiring only R (>= 3.4) and Rcpp (>= 0.11.2) (note:
compiling source packages may require the pre-installation of Rtools on Windows
and Xcode on Mac machines).
Detailed descriptions of the model and its potential can be
be found in the academic publication.